In vitro simulation of maternal-to-fetal transplacental transport of materials
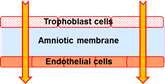
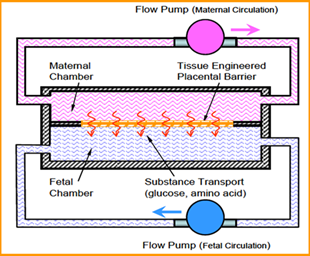
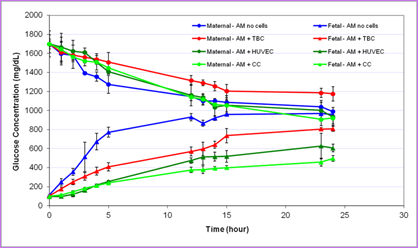
It is widely accepted that "fetal programming" for adulthood life takes place in utero and the intrauterine environment plays a critical role in child growth. The long-range anatomic and functional developments are largely affected by intrauterine metabolic engineering, especially during organogenesis in the first trimester. There is a consensus that maternal-to-placental supply of nutrients (e.g., glucose, fatty acids, amino acids) is determined by maternal nutrition and metabolism, transplacental concentration gradients, utero-placental and feto-placental blood flow, placental barrier (PB) structure and size, and by its transfer capabilities. Altered fetal growth, fetal growth restriction (FGR) or large-for-gestational age (LGA) baby conditions are associated with alterations in placental transport characteristics. Related global epidemics are obesity, diabetes and metabolic disorders. We have developed an in vitro simulator for maternal-to-fetal transplacental transfer of glucose across the tissue engineered in vitro PB model, including analysis of the contribution of sub-layers of cells of this model. Analysis of the transport results for the different components of the PB model revealed the following: (i) The AM is highly permeable to glucose compared to the cellular structures and can serve as a substrate for the co-culture even though it is thicker than the in vivo basal membrane; (ii) The EC are more resistive to glucose transfer than the TC; and (iii) The complete in vitro PB model is the most resistive to glucose transfer. The good correlation between the present in vitro results with existing in vivo data demonstrated the potential of this new approach, which can be extended to study various aspects of transplacental transfer, including medications, relevant to GDM or any problem related to in utero programming.
 |  |

